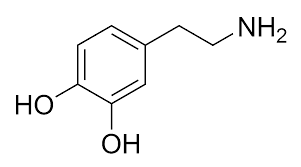
Lab Methods and Resources

in vitro electrophysiology
Equipped with Olympus BX51 microscope with optics for DIC and fluorescent imaging and electronics for patch-clamp recording. The LED light source can be filtered to various wavelengths for activation of optogenetics and cell type identification.
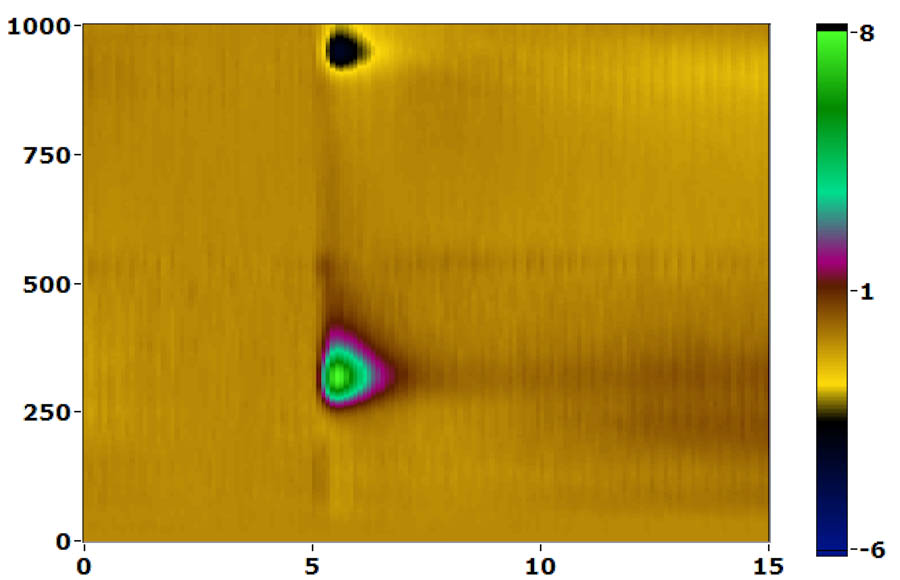
cyclic voltammetry
We do anesthetized FSCV using stereotaxic placement of stimulating and working electrodes and are currently implementing awake, behaving recording using chronically implanted electrodes. We have a dedicated Med-Associates operant chamber integrated with the FSCV system that includes time-stamped video recording to synchronize operant events, video and dopamine signals.
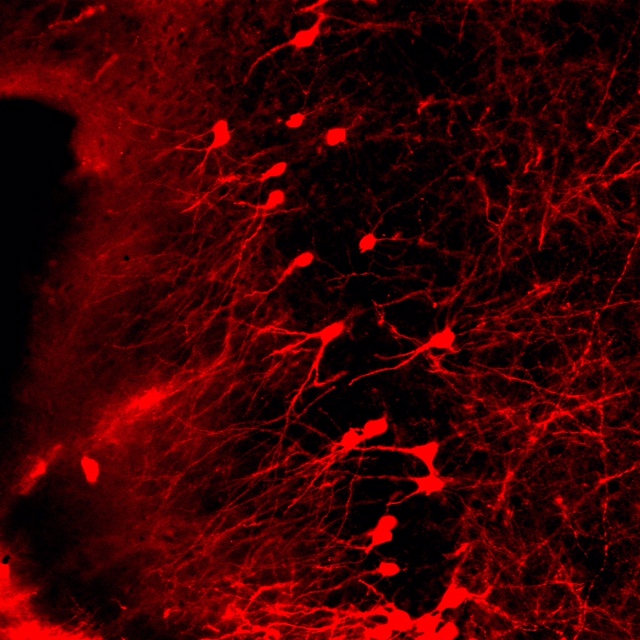
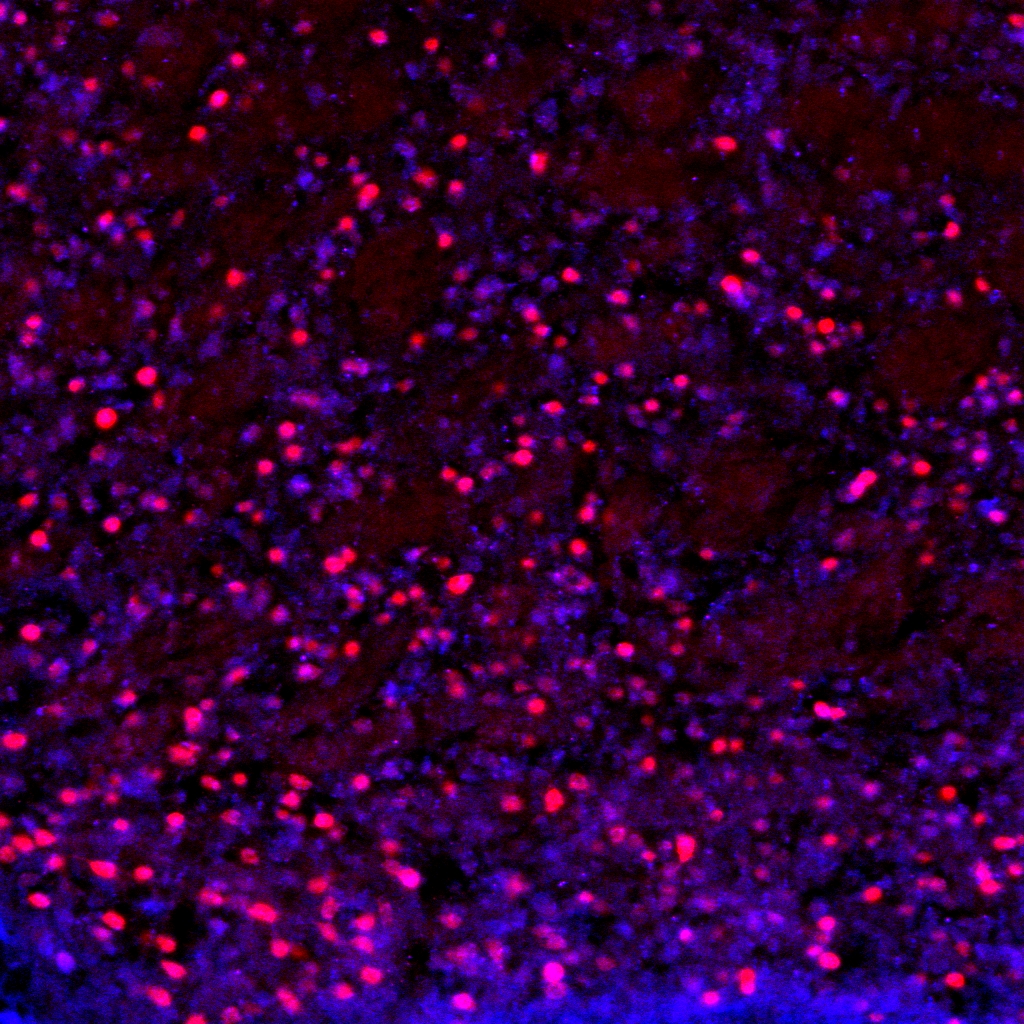
confocal microscopy
We share an Olymus confocal with two other labs on the floor. We use confocal in combination with both immunohistochemistry and genetically driven fluorescent reporters for visualization. We are interested in structural changes in discrete cell populations and circuit connectivity.

optogenetics and fiber photometry
We use optogenetics to selectively activate/inhibit specific cell populations and fiber photometry to measure neuronal activity in selected populations or to measure neurotransmitter release, including dopamine and acetylcholine. The goal is real-time measurement of neural activity that mediates on-going behavior in awake-behaving mice.

homecage operant cages
We use homecage operant paradigms in which the mice live in the operant chambers (modified homecage equipped with levers, food dispenser, etc.) in a closed economy. Using both genetic and pharmacological manipulation, we use these to characterize the effect of neural manipulations on behavioral adaptation to different environments. The resulting data are rich and amenable to fitting to computational models. We are building a custom homecage touchscreen system that, in addition to the standard equipment, will use a touchscreen to present a wider variety of stimuli and provide more flexible manipulanda options.

behavioral methods
We do a variety behavioral tests, including the accelerating rotarod, open field, forced swim test, sucrose preference, two-bottle conditioning, novel object recognition and simple maze behaviors, such as the plus maze for anxiety or the cross maze to dissociate place- and response-learning.
genetic mouse lines
We maintain an array of genetically altered mouse lines, including several tissue specific are-recombinase lines, lines with floxed genes (e.g., insulin receptor, dopamine D2, adenosine 2A, beta2 nicotinic receptor), several reporter lines (tdTomato, EGFP), various knockdown lines, and some genetic tools, including floxed channel rhodopsin 2, floxed halorhodopsin, floxed DREADDs and others.
Methods and Techniques
- behavioral assessment
- genetic manipulations
- fast-scan cyclic voltammetry
- patch-clamp electrophysiology
- immunohistochemistry
- confocal microscopy
- optogenetics
- fiber photometry
- quantitative PCR (qPCR)
Major Lab Equipment
- Neurophotometrics 12-channel, dual color fiber photometry system
- patch-clamp electrophysiology rig
- anesthetized FSCV rig
- 12 homage operant chambers
- 12 conventional operant chambers
- Columbus instruments Rotamex rotarod
- 24 radio telemetry running wheels
- 2 stereotaxic surgery stations with microinjection controllers and isoflurane anesthesia
- optogenetic equipment (LEDs, commutators)
- Olympus confocal microscope (shared)
- 3 Neurolucida-StereoInvestigator systems with Olympus BX51 microscopes and Ludl xyz stages, high resolution digital camera and full range of objectives/filters (shared)
- Leica CM1950 cryostat (shared)
- 2 Leica vibratomes (shared)
- Narshige vertical puller (PE-22)
- Sutter horizontal puller (shared)
- molecular biology wetlab
Additional Resources
- Queens College Biology core (qPCR, FACS, additional microscopy)
- Center for Computational Infrastructure for the Sciences (CCIS), Queens College
- CUNY High Performance Computing Center (CSI, Staten Island)
- Advanced Scientific Research Center (ASRC), imaging facility